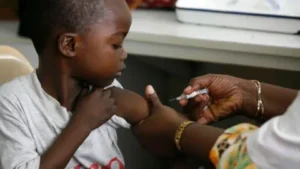
In a groundbreaking development within the realm of healthcare, the first malaria treatment specifically designed for infants and very young children has received approval for use. This vital milestone marks an important stride in the global fight against malaria, particularly affecting some of the world’s most vulnerable populations. Expected to be distributed across African nations in a matter of weeks, this new treatment promises to address a significant gap that has existed in pediatric healthcare.
Historically, infants and young children have been treated with formulations intended for older children. Such practices raised concerns, as these medications, particularly at prescribed doses for larger bodied children, posed a risk of overdose in smaller, developing infants. The lack of a tailored treatment for babies has compounded the issue of malaria, a disease that claimed approximately 597,000 lives in 2023 alone, with many of these fatalities occurring in children under the age of five. Notably, around 75% of these deaths were reported in Africa, underscoring the urgency of developing appropriate interventions for this demographic.
The recently approved medication, referred to as Coartem Baby or Riamet Baby in some regions, represents a collaboration between the pharmaceutical company Novartis and the Medicines for Malaria Venture (MMV), a not-for-profit organization. The initiative has garnered support from various international bodies, including the British, Swiss, and Dutch governments, the World Bank, and the Rockefeller Foundation, demonstrating a concerted global effort to combat the disease. Trials for the medication involved eight African nations that are expected to be among the initial recipients of this new treatment.
Vas Narasimhan, the CEO of Novartis, emphasizes the significance of this approval, highlighting the long-standing commitment of the company to advance scientific breakthroughs in the field of malaria. His perspective aligns with those of health experts who assert that the introduction of a suitable malaria treatment for babies is a pivotal moment in public health, especially in areas severely affected by the disease.
With 76% of malaria-related deaths occurring in children under five years old, having an approved treatment specifically formulated for uniform infant weight is crucial for ensuring their safety and elevating the chances of successful treatment outcomes. The development not only represents a medical advancement but also a step toward equity in healthcare accessibility, as Novartis plans to distribute the medication on a largely not-for-profit basis.
Martin Fitchet, the CEO of MMV, recognizes that such developments are essential to mitigate the appalling toll that malaria takes on countless lives, primarily among children. He reflects the beliefs of many in the medical community that with increased resources and emphasis on the matter, malaria can be effectively eradicated. The sentiment resonates strongly, particularly when considering the broader public health implications of such a breakthrough: it can significantly reduce mortality rates and improve the health landscape for the most vulnerable populations.
In essence, the approval of Coartem Baby heralds a new frontier in the battle against malaria, providing healthcare practitioners with a critical tool to safeguard the health and well-being of infants suffering from this deadly disease. Experts from various fields, including Dr. Marvelle Brown from the University of Hertfordshire, articulate the transformative potential of the drug in saving young lives. The initiative stands as a testament to the joint endeavors of scientific, governmental, and humanitarian sectors aimed at delivering healthcare solutions to the most needy. Through ongoing commitment and collaboration, the vision of a malaria-free future becomes increasingly attainable.