Recent reports from the UK’s Health Security Agency (UKHSA) have revealed concerning statistics around botulism, a potentially deadly condition, indicating that 38 cases have been documented across various regions in England over the past six weeks. This alarming trend is suspected to stem from the use of counterfeit Botox-like substances in cosmetic procedures. The severity of botulism is tied not only to its rare occurrence but also to the life-threatening consequences associated with toxins generated by the bacteria Clostridium botulinum, which are typically involved in these procedures.
The geographical scope of the reported cases extends through areas including the East, East Midlands, and North East of England. The UKHSA has made an urgent appeal to consumers seeking aesthetic treatments to ensure that their chosen practitioners are duly qualified and that the products being administered are appropriately licensed for use. This lack of verification poses significant risks to individuals opting for cosmetic enhancements, underlining an important advice point for the public.
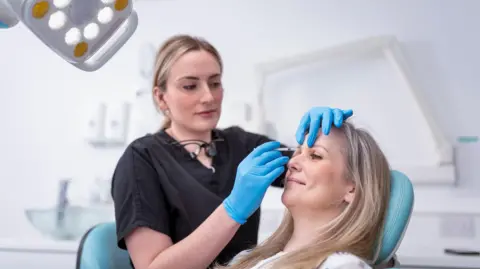
Botulinum toxin injections, commonly marketed under the brand name Botox, are frequently employed to diminish signs of aging such as wrinkles and fine lines. However, the toxic nature of the substance used means that improper usage can lead to severe health issues, including botulism. In the instances recently documented, patients displayed symptoms such as difficulties in swallowing, slurred speech, and breathing challenges, necessitating medical respiratory support. Other common symptoms of botulism may include drooping eyelids and double vision, demonstrating the dire potential side effects of not receiving treatment in a professional setting.
Dr. Gauri Godbole from the UKHSA emphasized the rarity of botulism in aesthetic medicine but reiterated the necessity of recognizing its serious implications. She noted that if anyone experiences symptoms indicative of botulism following cosmetic injections, they should seek assistance through the NHS 111 helpline promptly. It is crucial to highlight that Botox and its variants should only be prescribed following thorough consultations between patients and licenced healthcare providers.
Despite the current concerns surrounding counterfeit products, healthcare protocols require that a referral be made by qualified professionals, be they doctors or nurses, before any Botox treatment is administered. While the prescriber may delegate the actual injection process to another practitioner, they must still ensure those performing the procedure are qualified to do so.
Dr. Alison Cave, the chief safety officer at the Medicines and Healthcare products Regulatory Agency (MHRA), has warned of the dangers of acquiring botulinum toxins through unofficial channels, which significantly increases the risk of receiving unverified or falsified substances. These circumventions eliminate essential safety checks that ensure products meet established MHRA standards.
The Joint Council for Cosmetic Practitioners has echoed these concerns, reporting numerous incidents related to the illicit supply and use of unlicensed botulinum toxins. They advocate for individuals considering such procedures to conduct vigilant inquiries about the products in question, including the specific brand and dosage intended for the procedure. Patients should additionally reconfirm these details with their practitioner on the treatment day, ensuring the prescription issued is registered in their name.
To enhance safety when seeking cosmetic treatments involving Botox, the UKHSA put forth several recommendations. Practitioners must demonstrate their qualifications, maintain the proper hygiene by wearing appropriate protective gear, and uphold handwashing protocols. Future patients should also be provided with a thorough pre-treatment consultation to examine any pertinent medical conditions and the risks associated with the procedure. A well-delineated consent form should be discussed, ensuring that the patient fully understands the potential adverse effects before proceeding.
The implications of these warnings are significant for public health, as they highlight the necessity for thorough diligence and standardized protocols within the cosmetic surgery arena.