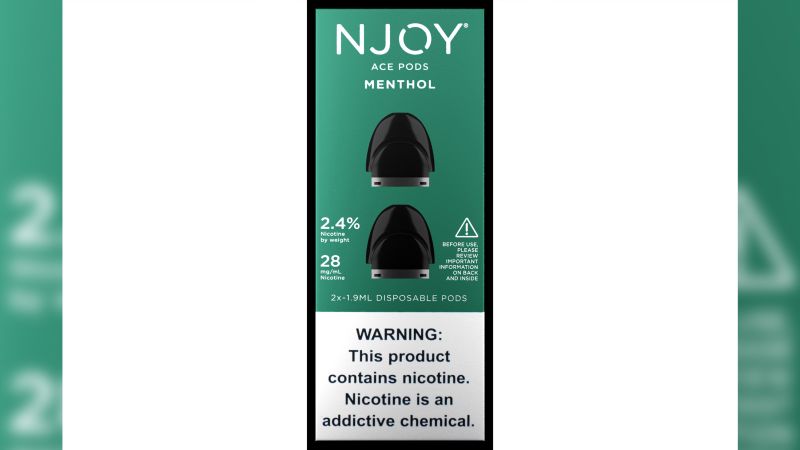
Good evening, I’m reporting live on the recent decision made by the US Food and Drug Administration regarding the authorization of four menthol vaping products. This decision marked the first time non-tobacco-flavored e-cigarette products received the agency’s approval.
The products in question, created by NJOY, an Altria company, include disposable e-cigarettes and nonrefillable pods used with the company’s reusable vaping device. Nicotine levels in these products range from 2.4% to 6%. Despite the authorization, the company did not provide a comment when approached by CNN.
The FDA clarified that the authorization does not imply these products are safe or FDA-approved, but rather that there is enough evidence to suggest they could benefit adult smokers seeking to transition from traditional cigarettes to e-cigarettes.
The decision has prompted criticism from pediatricians and anti-tobacco groups, who argue that the authorization could have harmful consequences, particularly for children. Organizations such as the Campaign for Tobacco-Free Kids, the American Cancer Society Cancer Action Network, and the American Academy of Pediatrics have expressed disappointment and concern over the FDA’s move.
On the other hand, the FDA mentioned that they have implemented strict marketing restrictions on these newly authorized products to minimize youth access and exposure. The agency also emphasized the importance of monitoring how the products are marketed and warned that failure to comply with regulations could result in the suspension or withdrawal of authorization.
The use of flavored e-cigarettes, especially those with high nicotine levels, poses a significant risk of addiction, particularly among young people. According to the Centers for Disease Control and Prevention, around 10% of high school-age adolescents and nearly 5% of middle school students currently use e-cigarettes.
While there has been a decline in the number of middle and high school students using e-cigarettes over the past five years, flavored e-cigarettes remain popular among teens and young adults. The FDA reported that over 23% of high school e-cigarette users nationwide prefer menthol-flavored products.
The decision to authorize menthol-flavored e-cigarettes has sparked concerns about potential harm to public health, especially among young individuals. As the FDA continues to navigate through millions of applications from e-cigarette companies, the debate surrounding the regulation of these products remains a pressing issue.