Cervical Cancer Screening Revolutionized with New Self-Collection HPV Tests
The first shipments of self-collection HPV tests for cervical cancer screening are currently being distributed to medical offices across the United States.
In May, the US Food and Drug Administration (FDA) approved the use of self-collected vaginal samples for cervical cancer screenings, offering patients an alternative to traditional HPV tests or Pap smears that typically require a speculum. This self-collection method allows patients to collect their own samples in a healthcare setting, including doctor’s offices, urgent care facilities, or pharmacy clinics.
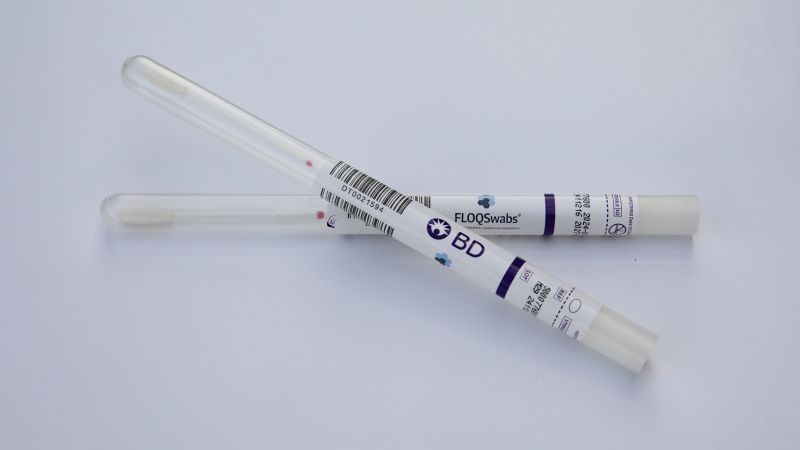
Two healthcare companies, Roche, a biotechnology firm, and BD, a medical technology company, already provide HPV tests suitable for self-collected samples. The majority of cervical cancer screenings focus on detecting human papillomavirus (HPV), the virus responsible for most cervical cancer cases.
According to BD, shipments of their self-collection HPV tests commenced on Thursday, while Roche expects to begin shipping its screening solution later this fall. Dr. Jeff Andrews, a board-certified gynecologist and vice president of Global Medical Affairs for Diagnostic Solutions at BD, expressed optimism about the new self-collection option, stating, “The hope is that providing a self-collection method will make screening more accessible for patients, leading to more women being screened.”
The Centers for Disease Control and Prevention (CDC) reports that over 11,000 new cases of cervical cancer are diagnosed each year in the United States, with approximately 4,000 women succumbing to the disease. Notably, it is estimated that nearly half of invasive cervical cancer cases are diagnosed in individuals who have never been screened, and around 10% of cases occur in those who have not been screened in the past five years.
Dr. Andrews highlighted the successful adoption of self-collection methods in other countries, noting, “In other countries in Europe, Australia, New Zealand, we know that adding the option of self-collection has reached some of those women who were previously under screened. So that’s the main goal, is to develop a methodology that would appeal to persons with a cervix who would otherwise not be screened.” He added that almost 30% of women in the U.S. are either unscreened or under-screened, with two-thirds of cervical cancers diagnosed in women who did not receive timely screenings.
The US Preventive Services Task Force recommends cervical cancer screening via Pap tests every three years for women aged 21 to 29 and combinations of HPV and Pap tests for women aged 30 to 65. Traditional methods of sample collection often involve discomfort, with some patients finding the speculum intrusive.
Cervical cytology evaluates cervical cells for abnormal changes, while the HPV test detects high-risk HPV types capable of causing cervical cancer. It is estimated that about 80% of people will contract an HPV infection at some point in their lives. HPV comprises over 150 viruses, some of which are high-risk and linked to several cancers, including cervical cancer. While most HPV infections resolve independently within two years, persistent infections can lead to serious health issues.
In a May news release following the FDA’s approval for HPV self-collection testing, Dr. Karen E. Knudsen, CEO of the American Cancer Society, stated, “Almost all cervical cancers are caused by persistent infection with certain types of HPV. Self-collection can expand access to screening and reduces barriers, which will give more people the opportunity to detect, treat, and ultimately survive cancer.”
The discomfort associated with traditional cervical cancer screening has led many to avoid recommended Pap tests. Research indicates that the perception of pain during Pap tests can significantly lower the likelihood that women will pursue their first screening. Experts suggest that minimizing discomfort may lead to increased participation in screening programs.
Dr. John Vullo, chairman of obstetrics and gynecology at Catholic Health’s Good Samaritan University Hospital, emphasized the critical need for accessible screening options. He stated, “If we can increase screening, we can reduce the incidence of this cancer. Having another modality or choice to make screening more accessible and available is important especially for our underinsured and underserved populations. Self-screening offers this extra choice for women.”
Dr. Vullo also highlighted the importance of provider supervision when using self-collection methods, ensuring patients understand the testing’s benefits and limitations. He stated, “This will allow us to offer them another modality to screen for cervical cancer while still giving them the benefit of speaking with a provider to discuss other health issues that are part of the annual well woman exam.”
Patients will need their healthcare providers to order the self-collection tests, ensuring they receive proper guidance on the results. The tests will be shipped to the patient’s doctor’s office, where they can use a swab to collect a sample, which is then sent to a laboratory for analysis.
While currently available in healthcare settings, companies hope to expand self-collection kits for at-home use. BD is collaborating with the FDA to support the home use of its self-collection tests.
Additionally, another company, Teal Health, has developed an at-home cervical cancer screening device known as the Teal Wand, which received “breakthrough device” status from the FDA in May, expediting its review process.
Regardless of the setting, cervical cancer screening is vital, especially since early-stage cases typically present no signs or symptoms. Advanced cervical cancer can cause abnormal bleeding and unusual discharge, necessitating various treatments, including surgery, chemotherapy, and radiation therapy.
According to the CDC, vital steps for preventing cervical cancer include receiving the HPV vaccination, abstaining from smoking, using condoms during sex, attending regular screenings, and promptly following up if screening results are abnormal.